Increasing efforts are devoted to implement immunotherapies for the treatment of acute myeloid leukemia (AML). Recently, natural killer (NK) cell-based immunotherapy has shown initial encouraging results. However, which patients are most likely to benefit from NK cell-based treatments remains poorly understood. Here, we investigate the responses of NK cells to ex vivo co-cultures with 55 primary AML samples using single-cell transcriptomics. We identify a subgroup of AML patients eliciting strong responses in NK cells and define the molecular programs in NK cells and other microenvironmental cell types triggered by diverse AML cells.
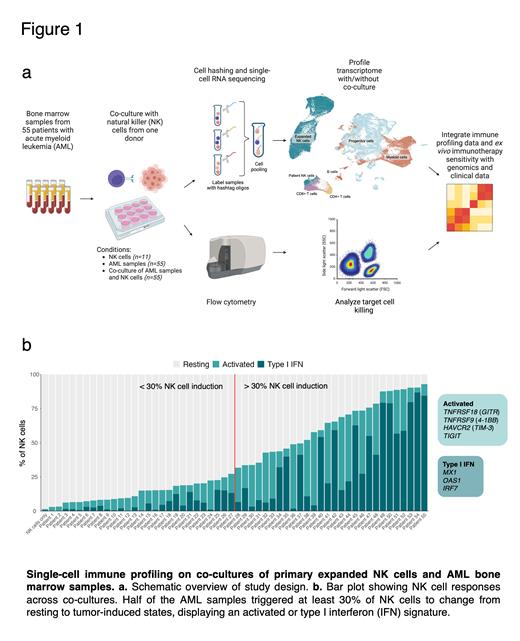
We performed functional single-cell immune profiling on co-cultures of primary expanded NK cells and 55 bone marrow (BM) samples from AML patients at diagnosis. We used NK cells expanded using irradiated K562-41BB-L-mbIL-21 feeder cells, resulting in a product commonly used in clinical trials. All expanded NK cells were from one single healthy donor displaying an HLA-C1C2 allotype.We investigated NK cell-treated and untreated cultures through multiplexed single-cell RNA sequencing (scRNA-seq) to dissect adaptive changes in both NK and AML BM cells upon their interaction. In total, we investigated 121 samples through scRNA-seq: 11 monocultures of expanded NK cells, 55 AML BM cells and 55 co-cultures of AML BM cells and expanded NK cells. scRNA-seq yielded 95,715 high quality cells (791 cells per sample on average), including 31,882 NK cells (483 cells per sample on average) and 63,833 AML BM cells (580 cells per sample on average). Unsupervised clustering of the cells revealed distinct clusters separating expanded NK cells from AML cells (Fig. 1a). We studied the sensitivity of leukemic cells to NK cytotoxicity using high-throughput flow cytometry. We further integrated the results with genetic and clinical data of the AML patients to characterize the mechanisms of action driving the transcriptomic responses and leukemic cell susceptibility to NK cell killing.
After 24 h co-culture, scRNA-seq revealed that AML BM cells induced distinct gene expression states in the NK cells. The observed tumor-induced NK states were predominantly enriched in co-cultured samples and not found in NK cell monocultures which mostly consisted of resting-state NK cells. Across the co-cultures, half of the AML samples triggered at least 30% of NK cells to change from resting to tumor-induced states (Fig. 1b). Out of the 28 AML samples causing the strongest NK cell induction, 54% primarily triggered a type I interferon (IFN) signature, characterised by expression of genes encoding MX1, OAS1, and IRF7. In contrast, the other 46% mainly induced an activated phenotype, including expression of TNFRSF18 ( GITR), TNFRSF9 ( 4-1BB), TIGIT and HAVCR2 ( TIM-3). Killer cell immunoglobulin-like receptors-HLA ligand mismatchesare well known causes of NK alloreactivity. Thus, we explored whether HLA-C matched (C1C2, n=24) and mismatched (C1C1 or C2C2, n=28) AML samples influenced NK cell responses. Interestingly, no significant difference in the NK cell induction states was observed between matched and mismatched samples (Mann-Whitney p = 0.57). NK cell treatment also induced responses in the patients' own T cells. Both CD4+ and CD8+ T cells upregulated IFN-ɣ and type I IFN genes upon co-culture. The type I IFN signature in CD8+ T cells was mainly found in AML samples triggering strong NK cell induction. CD8+ T cells also upregulated cytotoxicity genes including FASLG and TNFSF10 (TRAIL), along with inhibitory molecules such as LAG3, suggesting that NK cells can promote effector functions of T cells.
Our results suggest that AML BM cells from different patients trigger a heterogeneous response in expanded NK cells. Only in half of the cases, the leukemic BM cells induced substantial activation of NK cells. The observed variation in NK states was not determined by HLA-C allotypes, suggesting that other tumor cell features are responsible for the responses. This underscores the importance of understanding the molecular drivers of tumor and NK cell interaction. Our data additionally show that NK cell-based immunotherapy may be able to promote the functionality of the patient's own T cells, demonstrating how our ex vivo modeling approach can help to refine the mechanism of action of immunotherapies. Our work paves the way for identifying subgroups of AML patients responding to NK cell-based immunotherapies.
Disclosures
Lee:Avidicure B.V.: Consultancy, Current equity holder in private company, Research Funding; Kiadis Pharma, a Sanofi Corporation: Consultancy, Patents & Royalties: licensed through Nationwide Children's Hospital. Mustjoki:Pfizer: Research Funding; BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Dren Bio: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal